|
A CSL
lattice by definition has coincidence points in both lattices; the
CSL points thus are always O-lattice points, too. The converse is
not necessarily true (as we already have
seen in the example): |
|
 |
The O-lattice of two crystals in a
CSL orientation thus must include the CSL lattice points as
O-lattice points. This O-lattice, however, may also have
additional O-lattice points - all we can deduce at this point is that
the CSL lattice points must be a subset of the O-lattice points which belong
to the O-lattice that includes the specific CSL orientation. |
|
 |
We know that the CSL points are
O-points which are always of the same equivalence type - they are
lattice points, to be precise. In other words, the O-lattice belonging
to a certain CSL lattice, if drawn into the coordinate system of one of
the crystals is periodic in this reference
system. |
|
 |
This is not a
general property of an O-lattice - in general, every equivalence point
defined by an O-point could be
different from all the others and there would be no periodicity. |
 |
This is best visualized by drawing
all equivalence points encountered for a given O-lattice (which, of
course, always has infinitely many points) into the unit cell of one of the
crystals - we obtain the so-called reduced
O-lattice. |
|
 |
For a periodic reduced O-lattice, there would be a
finite number of equivalence points; a
non-periodic lattice would lead to an
infinite number of equivalence points in
the reduced O-lattice. |
|
 |
Lets look at some examples: |
|
|
|
|
|
|
 |
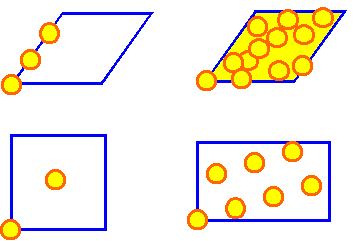
Shown are elementary cells of one lattice (blue)
with the equivalence points occurring in the O-lattice drawn in. In
three cases the O-lattice would be periodic; in the case in the upper
right, it would be non-periodic |
|
|
|
 |
Periodic O-lattices are
clearly special; and it is self-evident that every CSL orientation must
correspond to a periodic O-lattice. But there
is more. |
|
 |
At any O-lattice point in a
periodic O-lattice, we have a certain arrangement of the crystal atoms
around that point, a specific pattern.
Since in a periodic O-lattice there are only a finite number of
different equivalence points, there is only a finite number of distinct
patterns, too. |
|
 |
An individual
pattern is called a pattern element. There
are as many pattern elements as there are equivalent points in the reduced
O-lattice. |
|
 |
This is a crucial concept in
O-lattice theory, unfortunately it is not explained very well in
Bollmanns book. Let's see what is meant by pattern: |
|
|
|
|
|
|
|
|
|
|
 |
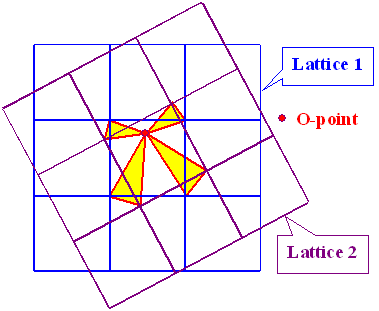
Shown are two lattices (blue and
magenta which are superimposed) and one O-point (red). A representation of the geometry of the atoms that you
may put into the lattices is given by the yellow triangles. They are simply
constructed by connecting the lattice points of the two lattices
"around" the O-point with the O-point. |
|
 |
The picture also demonstrates (but
does not prove) an universal theorem: Any
O-point can be chosen as the origin
for the transformation that produces lattice 2 from lattice 1
(here it is a simple rotation). |
 |
In a non-periodic O-lattice,
the representation of patterns in the way shown is different at any
O-point - this is also rather difficult to draw. |
|
 |
This is where O-lattice theory
gets hard to illustrate. Nobody surpasses Bollmann who provides complicated
drawings of patterns (done by hand!) in his book,
one example is shown in the
link. |
 |
The question now is: Which
orientations provide periodic
O-lattices? It appears that there is no simple formula coming up with
transformation matrices or angles for rotations that produce periodic O-lattices. We have to go the other
way and ask two questions for any possible
orientation: |
|
 |
Is the corresponding O-lattice
periodic? |
|
 |
If yes, how many pattern elements
(= N) are contained in the reduced O-lattice? |
 |
What we want is N as a
function of some misorientation angle for some simple geometries. This needs
some numerical calculations; lets look at the results for rotations on the
{110} planes of cubic crystals |
|
 |
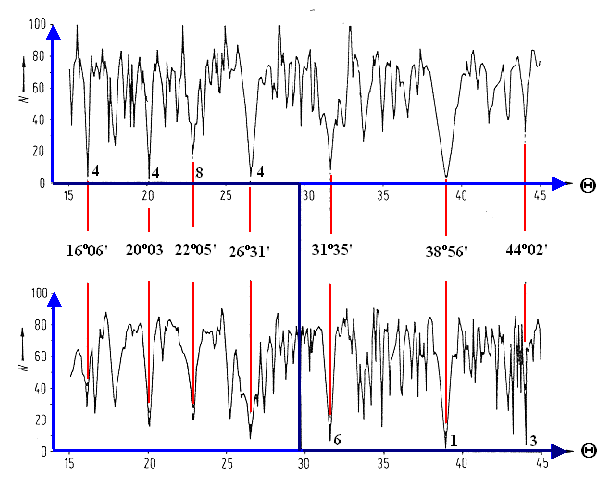
The following picture shows N
as a function of the misorientation angle: |
|
|
|
|
|
|
|
|
|
|
 |
This again is one of Bollmanns trickier
pictures (with some color added), because it is only understandable if you read and understood much
of what has been said before in his book (it is neither explained what the
difference is between the two curves - they have after all an identical
coordinate system, nor what the bold lines (here dark blue) implicate). |
|
 |
Well, the N-values are given
for two independent kinds of
transformations which both include the same rotation T (upper and lower curve), but one
inlcudes a so-called "unimodular transformation" in addition. The one
with the smallest determinant which, as
we have seen, is the one you should use, changes from the upper curve to
the lower curve at T = 30o
and this explains the bold (or dark blue) lines. Since the two curves are
different, you now see that it matters, indeed, which transformation matrix you
pick. |
|
 |
Don't worry; it is not necessary to
understand that in detail. Just acknowledge, that N can be computed and
that unambiguities with respect to different transformation matrices can be
dealt with somehow. |
|
 |
Also note that the "real"
curve would be a fractal with N = ∞ for
most values; it is smoothed here by only counting the equivalence points in
100 "pixels" of the O-lattice (so N
³ 100 applies) and stopping the numerical
procedure after some time if it does not turn periodic anyway. |
 |
It is clear that there are several
"special" orientations for this geometry with small values of
N. This looks good, however, we are not yet done. We are really looking
for O-lattices that are periodic on a short scale, i.e. the patterns
should repeat after a short distance. This requires three ingredients: |
|
 |
Periodicity as a starter, i.e. N is finite
(or in reality e.g. N < 100 for numerical calculation). |
|
 |
Small
values of N, because the pattern repeats after N steps
- the larger N, the longer it takes for a repetition. To give an
example: For N = 10 you have to go out 10 lattice constants of
the O-lattice before the same pattern is encountered again. |
|
 |
This immediately calls for small lattice constants of the O-lattice,
too. Or, to be more general, for small volumes
VO of the O-lattice cells. |
 |
The real measure for
the periodicity of the O-lattice patterns is therefore not N, but
the density N' of periodic
equivalence points given by |
|
|
|
|
|
|
|
|
 |
With |T| meaning the determinant of the transformation
matrix, since VO = 1/ |T| follows from basic
matrix algebra together
with the definition of the
O-lattice. |
 |
Now comes a major point: N' is nothing but
the number of crystal units (volume of unit cells or lattice constants) per
period of the pattern because the unit of the O-lattice is always (for
periodic O-lattices) an integer number of the crystal units! |
|
 |
In other words: N' corresponds directly to the measure
of coincidence in the CSL model, the number Σ! In fact, the numerical values are identical in most
(but not all) cases: N' = Σ. |
|
 |
The O-lattice theory, however, is not only much more
general, but gives the recipes for calculating N' (or Σ). Try, for example, to find the CSL lattices
for orientations between, say a cubic and a monoclinic lattice: All you need
are the deformation matrices; the rest can be done for all possible cases by a
computer program. |
|
 |
Just one case in point: What happens for perfectly well
defined transformation matrices T, but with |T|
= 0? N' in this case will be ∞. |
|
 |
Lets look at an example: Rotation of cubic lattices by an
angle around a <110> direction: |
|
|
|
|
|
| Angle Θ |
|T| |
N |
N´ |
Σ |
| 10o 6,0´ |
0,031 |
4 |
129 |
129 |
| 13o 26,06 |
0,055 |
4 |
73 |
73 |
| 20o 3,0´ |
0,121 |
4 |
33 |
33 |
| 22o 50,4´ |
0,157 |
8 |
51 |
51 |
| 26o 31,6´ |
0,210 |
4 |
19 |
19 |
| 38o 56,6´ |
0,111 |
1 |
9 |
9 |
| 50o 28,6´ |
0,000 |
|
oo |
11 |
| 58o 59,6´ |
-0,030 |
1 |
33 |
33 |
| 70o 31,6´ |
0,000 |
|
oo |
3 |
|
|
|
|
|
 |
Two perfectly well defined rotations lead to |T| = 0; their Σ values are 11 and 3, respectively,
while N' is infinity! |
|
 |
This tells us that these particular orientations are much more special then implied by their Σ values: These orientations can be obtained by
simpler transformations matrices of lower rank and they correspond to grain
boundaries with a particular high degree of "fitting" and thus low
energy. |
|
 |
There is also a first real result: Σ11 boundaries should be rather common, and that's
what they really are. |
 |
We will not go into more details at
this point; but it should become clear that there is a lot of power behind the
O-lattice theory. |
|
 |
However, even at this stage, calculations become
tedious and need numerical methods. It would be most useful to implement the
basic equations in a computer program from now on - but I do not know if this
has been done. |
|
 |
And, always keep track of this: So far we have
only dealt with "small
deformation" boundaries and with high angle boundaries having a
periodic O-lattice. We are still
some distance away from a general boundary. |
 |
We now need to do the next step -
always, for easier understanding, in analogy to the CSL model of grain
boundary structures: |
|
 |
What happens if the orientation of the two
crystals (including arbitrary lattices and thus phase boundaries, too) is close
to, but not exactly at a "special" O-lattice orientation?
"Special" meaning a periodic O-lattice. |
|
 |
In other words, we are asking for possible
structural defects which can be superimposed and will change the (generally
non-periodic) O-lattice of an arbitrary boundary (which is always
defined) just the right amount to generate a periodic O-lattice with a
supposedly low energy? |
|
 |
This is the essentially the
same question we asked for crystals
close to, but not exactly at a " low Σ" orientation - but on a much higher level of
abstraction and with the possibility to deal with it quantitatively. |
© H. Föll