|
If we look at the infinity of
possible orientations of two grains relative to each other, we will find some
special orientations. In two dimensions
this is easy to see if we rotate two lattices on top of each other. |
|
 |
You can watch what will happen for a
hexagonal lattice lattice by
activating the link. |
 |
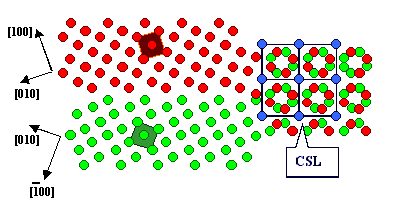
A so-called Moirée pattern develops, and for
certain angles some lattice points of
lattice 1 coincide exactly with
some lattice points of lattice 2. A
kind of superstructure, a coincidence
site lattice (CSL),
develops. A question comes to mind: Do these special coincidence orientations
and the related CSL have any significance for grain boundaries? |
 |
Lets look at our paragon of grain
boundaries, the twin boundary: |
|
|
|
|
|
 |
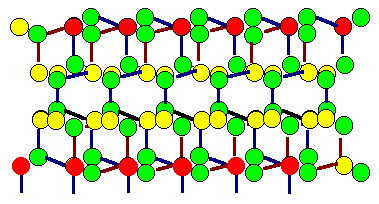
Shown are the two grains of the
preceding twin boundary, but
superimposed. Coinciding atoms (in the
projection) are marked red. However, this might be coincidental (excuse the
pun), because the atoms in this drawing are
not all in the drawing plane. Note that it is not relevant if the boundary itself is coherent or
not - only the orientation of the grains counts. |
 |
And once more, note that the lattice is not the crystal! We
are looking for coinciding lattice points -
not for coinciding atom positions (but this may be almost the same thing with
simple crystals). |
|
|
|
|
|
|
|
|
 |
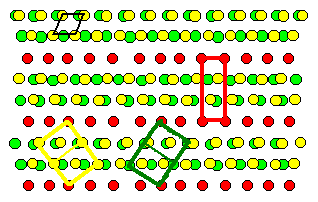
So in this picture the same situation is shown for the
fcc lattice belonging to the grain
boundary. Again, coinciding lattice points
are marked red and a (two-dimensional) elementary cell of the CSL is
also shown in red. The two (three-dimensional) elementary cells of the
fcc lattices are also indicated. |
 |
It is definite from this picture that the the twin boundary
belongs to the class of boundaries with a coincidence relation between the two
lattices involved. |
|
|
|
|
|
 |
From the animation in the
link above it was clear that many coincidence
relations exist for two identical two-dimensional lattices. In order to be able
to extend the CSL consideration to three dimensions and to generalize
it, we have to classify the various possibilities. We do that by the following
definition: |
|
|
|
|
|
Definition:
The relation between the number of lattice points in the unit cell of a
CSL and the number of lattice points in a unit cell of the generating
lattice is called Σ (Sigma); it is the unit cell volume of the
CSL in units of the unit cell volume of the elementary cells of the
crystals. |
|
|
|
|
|
 |
A given Σ
specifies the relation between the two grains unambiguously - although this is
not easy to see for, let's say, two orthorhombic or even triclinic
lattices. |
 |
If we look at the twin boundary
situation above, we see that Σtwin =
3 (you must relate the two-dimensional lattices her; one is pointed above
out in black!). For the three-dimensional case we still obtain Σ = 3 for the twin boundary, so we will call twin
boundaries from now on: S3 boundaries. |
|
 |
A Σ1 boundary
thus would denote a perfect (or nearly perfect) crystal; i.e. no boundary at
all. However. boundaries relatively close to the Σ1 orientation are all boundaries with only small misorientations called "small-angle grain boundaries"
- and they will be subsumed under the term Σ1
boundaries for reason explained shortly. |
|
 |
Since the numerical value of
Σ
is always odd, the twin
boundary is the grain boundary with the most special coincidence orientation
there is, i.e. with the largest number of coinciding
lattice points. |
 |
Next in line would
be the Σ5 relation defining the
Σ5
boundary. It is (for the two-dimensional case) most easily seen
by rotating two square lattices on top of each other. |
|
|
|
|
|
|
|
|
|
 |
This also looks like a pretty "fitting"
kind of boundary, i.e. a low energy configuration. |
 |
A
suspicion arises: Could it be that grain boundaries between grains in a
CSL orientation, especially if the Σ
values are low, have particularly small grain boundary energies? |
 |
The answer is:
Yes, but... . And the "but"
includes several problems: |
|
 |
Most important: How do we get an answer?
Calculating grain boundary energies is still very hard to do from first
principles (Remember, that we can't calculate melting points either, even
though its all in the bonds). First principles means that you get the exact
positions of the atoms (i.e. the atomic structure of the boundary and the
energy). Even if you guess at the positions (which looks pretty easy for a
coherent twin boundary, but your guess would still be wrong in many cases
because of so-called "rigid body translations"), it is hard
to calculate reliable energies. |
 |
So we are left with experiments. This involves
other problems: |
|
 |
How do you measure grain boundary energies? |
|
 |
How do you get the orientation relationship? |
|
 |
How do you account for the part of the energy that
comes from the habit plane of the boundary - after all, a coherent twin (habit
plane = {111}) has a much smaller energy than an incoherent one? |
 |
Getting experimental results appears to be rather
difficult or at least rather time consuming - and so it is! |
|
 |
Nevertheless, results have been obtained and, yes,
low Σ boundaries tend to have lower energies
than average. |
|
 |
However, the energy does not correlate in an easy
way with Σ; it does not e.g. increase
monotonously with increasing Σ. There might
be some Σ values with especially low energy
values, whereas others are not very special if compared to a random
orientation. |
 |
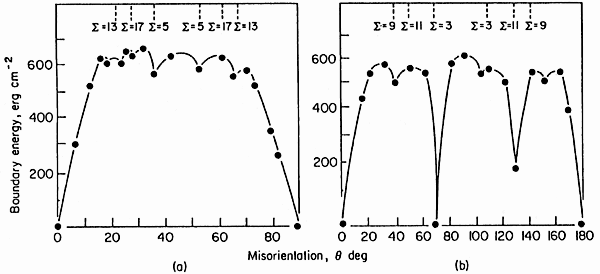
The result of (simple) calculations
for special cubic geometries are shown in the picture: |
|
|
|
|
|
|
|
|
|
 |
Shown is the calculated (0oK)
energy for symmetric tilt
boundaries in Al produced by rotating around a <100> axis
(left) or a <110> axis (right). We see that the energies are
lower, indeed, in low Σ orientations, but
that it is hard to assign precise numbers or trends. Identical Σ values with different energies correspond to
identical grain orientation relationships, but different habit planes of the
grain boundary. |
 |
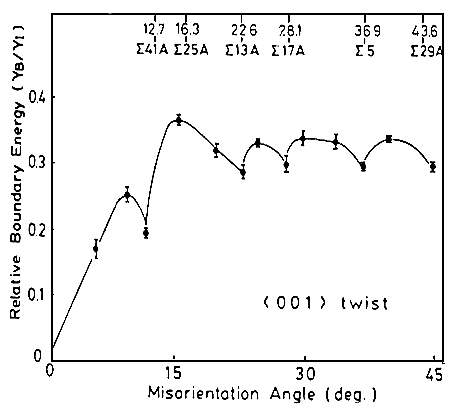
The next figure shows grain boundary energies for
twist boundaries in Cu that were actually measured by Miura et al. in
1990 (with an elegant and ingenious, but still quite tedious method). |
| |
|
|
|
|
|
|
|
|
 |
Clearly, some Σ
boundaries have low energies, but not necessarily all. |
 |
Nevertheless, in practice, you tend
to find low Σ boundaries, because (probably)
all low energy grain boundaries are boundaries with a defined Σ value. And these boundaries may have special
properties in different contexts, too. |
|
 |
The link shows the
critical current density (at
which the superconducting state will be destroyed) in the high-temperature
superconductor YBa2Cu3O7 with
intentionally introduced grain boundaries of various orientations and
HRTEM image of one (facetted) boundary. It is clearly seen that the
critical current density has a pronounced maxima which corresponds to a low
Σ orientation in this (Perovskite type)
lattice. |
 |
However, despite this or other direct
evidence for the special role of low Σ
boundaries, the most clear indication that low Σ boundaries are preferred comes from innumerable
observations of a different nature altogether - the observation that grain
boundaries very often contain secondary defects with a
specific role: They correct for small deviations from a special low Σ orientation. |
|
 |
In other words: Low Σ
orientations must be preferred, because otherwise the crystal would not
"spend" some energy to create defects to compensate for
deviations. |
 |
If we accept that rule, we also have
an immediate rule for preferred habit planes of the boundary: |
|
 |
Obviously, the best match can be made
if as many CSL points as possible are contained in the plane of the
boundary. This simply means: |
|
 |
Preferred grain boundary planes are the closest packed planes of
the corresponding CSL lattice. |
 |
We will look at those grain boundary
defects in the next sub-chapter. |
© H. Föll