|
|
 |
So-called "twin boundaries" are the most frequently
encountered grain boundaries in Silicon, but also in many other fcc
crystals. This must be correlated to an especially low value of the interface
energy or grain boundary energy
always associated with a grain boundary. This becomes immediately
understandable if we construct a (coherent) twin boundary. The qualifier
"coherent" is needed at this point, we will learn about its meaning
below. |
|
 |
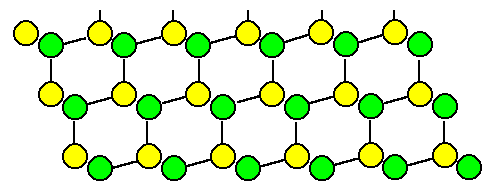
Lets look at the familiar <110> projection of the
diamond lattice: |
|
|
|
|
|
|
|
|
|
|
 |
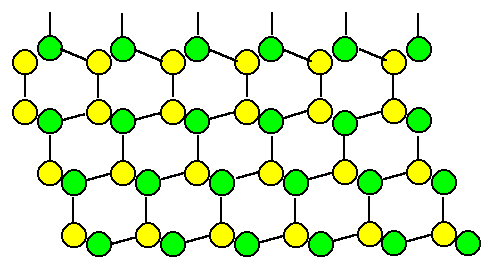
Now we introduce a stacking fault, e.g. by adding the next
layer in mirror-symmetry (structural chemists would call this a "cis" instead of a "trans" relation): |
|
|
|
|
|
|
|
|
|
|
 |
If we were to continue in the old stacking
sequence, we would have produced a stacking fault. However, if we continue with
mirror-symmetric layers, we obtain the following structure without changing any bond lengths or bond
angles: |
|
|
|
|
|
|
|
|
|
 |
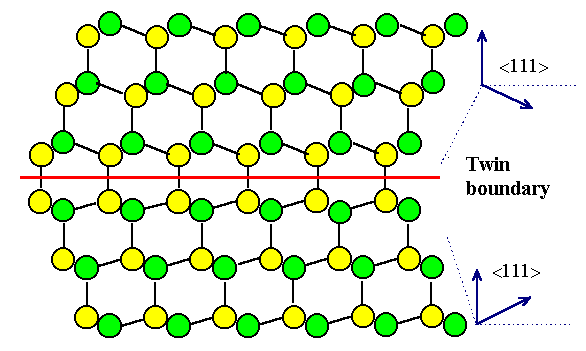
We generated a (coherent) twin
boundary! This is obviously a special
grain boundary with a high degree of symmetry. |
 |
Now let's try to describe what we did
in general geometric terms. To describe the
twin boundary from above or just any
boundary geometrically, we look at the general case illustrated below: |
|
|
|
 |
We have two arbitrarily oriented grains joined together at the
boundary plane. We thus need to define the "arbitrary" orientation
and separately the boundary plane. |
|
|
 |
Choosing grain
I as reference, we now can always "produce" a arbitrary
orientation of the second grain by "cutting" a part of grain I
off - along the boundary plane - and then rotating it by arbitrary angles
α, β and
γ around the x-,
y- and z- axis (always defined in the reference
crystal, here grain I). |
|
|
 |
Of course, the grain II thus produced would not fit
together anymore with grain I. So we simply remove or add grain
II material, until a fit is produced. |
|
|
 |
Alternatively, we could simply rotate the
second crystal around one angle α if we pick a suitable polar vector for that. |
|
|
 |
Specifying this polar (unit) vector will need two numbers or its direction - e.g. two angles
relative to the boundary plane or any other reference plane, and one
number for its length specifying the angle of rotation. |
|
|
 |
In either way: three
numbers then for the orientation part. |
|
|
|
|
|
|
 |
The boundary then is defined by
5 parameters: The three rotation
angles needed to "produce" grain II, and two parameters to define the boundary plane by its
Miller indices {hkl} in the coordinate system of the reference grain
I. |
|
 |
Why do we need only two parameters to define the boundary plane? After
all, we usually need three Miller indices
{hkl} to indicate a plane in a crystal? |
|
 |
Good question! We need only two numbers in this case because here a unit vector with the right orientation given by
{hkl} is sufficient - the length, likewise encoded in {hkl}, does
not matter. Since you need only two angles
between a unit vector and the coordinate axis' to describe it unambiguously,
two numbers are enough, even so they cannot
be given straightforward in {hkl} terms. |
|
 |
The third angle is then always given by the Euler relation |
|
|
|
|
|
| sin2α +
sin2β +
sin2γ |
= |
1 |
|
|
|
|
|
|
|
 |
Again, Miller indices do not only
give the direction of a vector perpendicular to a crystallographic plane, but
also a specified length which contains the distance between the indexed
crystallographic planes - and that's why they need three numbers in contrast to a unit vector. |
 |
Thus, constructing a
simple (coherent) twin boundary, looking at it and generalizing somewhat, we
learned a simple truth: |
|
|
|
|
|
|
A (simple) grain boundary needs (at least)
5 parameters
for its geometric description |
|
|
|
|
|
|
 |
That was some basic geometry for a
simple grain boundary! For
real grain boundaries we must add the
complications that may prevail, e.g.: |
|
 |
The grain boundary is not flat (not on one plane),
but arbitrarily bent. |
|
 |
The grain boundary contains (atomic) steps and
other local "grain boundary defects". |
|
 |
The grain boundary contains foreign atoms or even
precipitates. |
|
 |
The grain boundary is not crystalline but consists
of a thin amorphous layer between the grains. |
 |
Since all those (and more)
complications are actually observed, we
must (albeit reluctantly) conclude: |
|
|
|
|
Grain boundaries are rather complicated defects!
|
|
|
|
 |
We can learn more about grain boundaries by
analyzing our (coherent) twin somewhat more. While we generated this defect by
adding {111} layers in a mirror-symmetric way, there are other ways of doing it, too: |
 |
We first consider the twin boundary as a pure
twist boundary. The recipe for creating a
twist boundary following the general recipe
from above is as follows: |
|
 |
Cut the crystal along a {111} plane (using
Volterras
knife, of course). |
|
 |
Rotate the upper part by 180° or 60°
around an axis perpendicular to the cut
plane (= "twist") |
|
 |
Weld the two crystals together. There will be no problem,
everything fits and all bonds find partners. |
 |
This procedure is shown below. We are forced to
conclude that we obtain exactly the same twin boundary that we
produced above! |
|
|
|
|
|
|
|
 |
Now lets look at the other extreme: We construct
our twin boundary as a pure tilt boundary.
The recipe for creating a tilt boundary
following the general recipe from above is as follows: |
|
|
|
|
|
|
|
|
|
|
 |
Cut the crystal along a {111} plane (using
Volterras knife,
of course) |
|
 |
Rotate the upper part by 70.53° around an axis
perpendicular to the drawing plane (=
"tilt" the grain); i.e. use a
<110> direction for rotating. |
|
 |
Now, however, we must fill in or remove material as
necessary. |
|
 |
Weld together. |
 |
This procedure is shown above; again we obtain
exactly the same twin boundary we had before! |
|
 |
This is not overly surprising, after all the symmetries of the
crystal should be found in the construction of grain boundaries, too.
|
|
 |
Well this is conceptually easy to grasp, it generates a major
problem in the mathematical analysis of the grain boundary structure. What you
want to do then is to generate a grain boundary by some coordinate transformation of one grain, and than
analyze its properties with respect to the necessary transformation matrix. If there are several (usually
infinitely many) possible matrices, all producing the same final result, you
have a problem in picking the "right" one. We will run into that
problem in chapter 7.3. |
|
|
|
 |
Now lets look at the energy of the twin boundary
and see if we can generalize the findings. We are interested in answering the
following questions: |
|
 |
Is the grain boundary energy (= the energy needed to generate
1 cm2 of grain boundary) a function of the 5
parameters needed to describe the boundary? The answer, of course, will be
yes, so now we ask: |
|
 |
For a given orientation,
could a small change in the three angles describing that orientation induce
large changes in the energy? Asking a bit more pointedly: Are there
orientations leading to boundaries with especially small or large energies?
Are there favorable and unfavorable
orientations? |
|
 |
For a given orientation,
are there possibilities to minimize the energy, e.g. by changing the boundary
plane? Are there favorable and unfavorable
planes? |
 |
We will be able to answer these question to some
extent by using our twin boundary. First lets look at the energy of the
(coherent) twin boundary as shown above. |
|
 |
We would expect a rather small energy per unit
area, because we did not have to change bond lengths or angles. We should
expect that the energy of a (coherent) twin boundary should be comparable to
that of a stacking fault. |
|
 |
It is hard to imagine a boundary with lower energy and this
explains why one always finds a lot of twin boundaries in cubic (and
hexagonally) close-packed crystals |
 |
Now lets generate a twin boundary with the
"twist" or "tilt" recipe, but with twist or tilt angles slightly off the proper
values. Lets assume a twist angle of e.g. 58° instead of
60°. We then make a boundary with a similar, but distinctly
different orientation. |
|
 |
Try it! Can't be done. Nothing will fit any more; a lot of
bonds must be stretched or shortened and bent to make them fit. No doubt, the
energy will increase dramatically. In other words: |
 |
The energy of a grain
boundary may dependent very much on the
precise orientation relationship |
 |
Next lets imagine the generation of a twin
boundary where the twist or tilt angle is exactly right, but where the cut-plane is slightly off {111}. The result
looks like the schematic drawing below: |
|
|
|
|
|
|
|
|
|
 |
Again, we have a hard time with the welding procedure. Some
atoms will find partners with a slight adjustment of bonds, but other are in
awkward positions, e.g. the atoms colored blue in the above illustration.
The energy will be much higher than for a {111}
plane - for sure. In other words: |
 |
The energy of a grain
boundary may dependent very much on the
Miller Indices of its plane |
|
 |
We now can understand the meaning of the qualifier "coherent" in connection with a twin
boundary: Only twin boundaries on {111} planes are simple boundaries;
they are called coherent to distinguish them from the many possible
incoherent twin boundaries with planes
other than {111}. |
|
|
|
 |
From the last observation we can
easily deduce a first recipe for optimizing, i.e. lowering, the energy of a
given grain boundary: |
|
 |
Decompose the grain boundary plane into planes with low
energies. If that cannot be done, form at least large areas on low-energy
planes and small areas of connecting high-energy planes. In other words,
approximate the plane by a zig-zag configuration of planes. |
|
 |
This process is called facet forming or
facetting, the boundary
plane forms facets. This is
illustrated below. |
|
|
|
|
|
|
|
 |
Grain boundary on low-energy plane (i.e. a
{111} plane for a twin boundary). The {111} planes are indicated
by the dashed lines |
 |
Grain boundary on high -energy plane |
 |
Energy optimization by facetting on {111}.
The total area increases somewhat, but the energy decreases. |
|
|
|
|
 |
This is an important insight
with far-reaching consequences: We need no longer worry very much about the
grain boundary plane! It is always possible to optimize the energy by
facetting. |
|
 |
Facetting involves, of course, the movement of atoms. However,
only small movements or movements over small distances are needed, so facetting
is not too difficult if the temperatures are not too low. So the crystal has an option - it can change the
boundary plane by moving a few atoms around. |
|
 |
Experience, too, seem to show that boundary planes are not
very important: Grain boundaries, as revealed by etching or other methods, are
usually rather curved and do not seem to "favor" particular planes -
with the exception of coherent twins. This, however, is simply an illusion
because the facetting takes place on such a small scale that it is not visible
at optical resolution. |
 |
We now must deal with the relation between the relative orientation and the grain
boundary energy. Two questions come to mind: |
|
 |
Are there any other low-energy
orientations besides the rotations around <111> or
<110> that produces the low-energy twin boundary? |
|
 |
Is there a way to minimize the energy of a grain boundary that
is close to, but not exactly in a low energy orientation (some analogon to the
facetting of the planes)? |
|
 |
This is not an easy question, because the crystal does not have an option of changing the orientation
relationsship. In principle it would be possible, but it would imply
moving a lot of atoms - all the atoms inside a grain - and that is rather
unlikely to occur. |
 |
Answering these questions will lead
us to an important theory for the structure of grain boundaries (and phase
boundaries) which will be the subject of the next sub-chapter. |
|
|
© H. Föll