 |
Now you must solve a simple looking
integral. There are several ways of doing that |
|
 |
- Find a good math book with lots of integrals and take the solution from
there (the "Bronstein", however, won't do)
- Do a sensible approximation and solve it yourself in a simple way
- Go all the way and solve it completely - if you can.
|
|
 |
Here we go the second route. |
 |
We use a Taylor expansion for
1/u around u0 because that's where
u is felt most critically - for large values of u
everything tends to be zero anyway. In full generality we have |
|
|
|
|
|
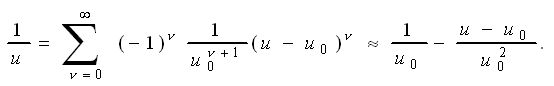 |
|
|
|
|
 |
If we keep it really simple, we could just use
the first term, having 1/u »
1/u0; but we will go one step beyond this and take |
|
|
|
|
|
|
|
|
|
|
 |
This gives us |
|
|
|
|
|
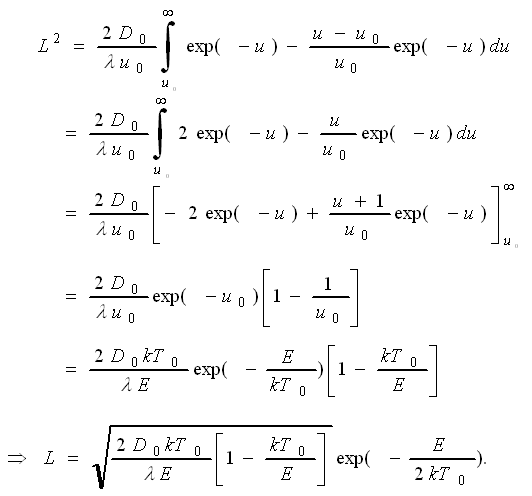 |
|
|
|
|
 |
The second term of the Taylor
expansion brought in the factor [1 –
kT0/E] and since kT0 «
E in all normal cases, it is indeed not very important. If we neglect it,
we may simply give the desired solution as |
|
|
|
|
|
| L = |
( |
2D0 · kT0
λ · E |
) |
1/2 |
· exp |
|
E
2kT0 |
|
|
|
|
|
 |
Now we can look at some typical cases
and see what this formula means. However, first we have to find the right
values for λ |
|
 |
For this we have to take the given values of the
initial colling rate which we call λ'and see
what λ values correspond to these cooling
rates. |
|
 |
The initial cooling rate λ' is the derivative of the T(t)
function at t = t0 = 0, we thus have |
|
|
|
|
|
d
dt |
(T0 · exp – λ · t |
| |
t = 0 |
= |
λ' = |
– λ ·T0
· exp –λ · t |
| |
t = 0 |
= – λ
·T0 |
|
|
|
|
|
|
 |
and obtain |
|
|
|
|
|
|
|
|
|
|
 |
The "–" sign cancels,
because our λ' must carry a minus sign, too,
if it is to be a cooling and not a heating rate. |
 |
Replacing λ by λ'/T0 yields the final formula:
|
|
|
|
|
| L = |
( |
2D0 · kT02
λ' · E |
) |
1/2 |
· exp |
|
E
2kT0 |
|
|
|
|
|
|
 |
We have to evaluate this formula for cooling
rates λ' given as (–) 1
oK/s, 10 oK/s, 50 oK/s,
104 oK/s, and activation energies of E =
1.0 eV, 2.0 eV, 5 eV. For D0 we take
D0 = 10–5
cm2s–1. |
|
 |
The result (including the [1 –
kT0/E] term is shown below |
|
|
|
|
|
|
|
|
|
 |
What can we learn from the formula
and the curves? |
|
 |
- The cooling rate is not all that important. Differences in the cooling rate
of a factor of 50 produce only an order of magnitude effect or less
since L is only proportional to (1/λ)1/2.
- The starting temperature T0 is slightly more
important than the activation energy E; both have the same weight
in the exponential, but T0 appears directly in the
pre-exponential while E enters only as square root.
- The pre-exponential factor D0 of the
diffusion coefficient is exactly as important as λ' and E in the pre-exponential factor
of the equation for L
|
|
 |
What can we do with the numbers? Quite simple:
- L gives you the average of the largest distance between some
point defect agglomerates, e.g. precipitates, because point defects farther
away than L from some nuclei cannot reach it and must form their
own agglomerate.
- The average number of point defects in an agglomerate divided by
L3 gives a lower limit for the point defect
concentration, because at least as many point defects as we find in an
agglomerate must have been in the volume L3.
|
|
|
|
© H. Föll
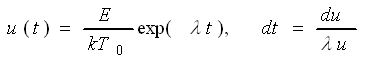
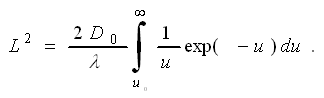
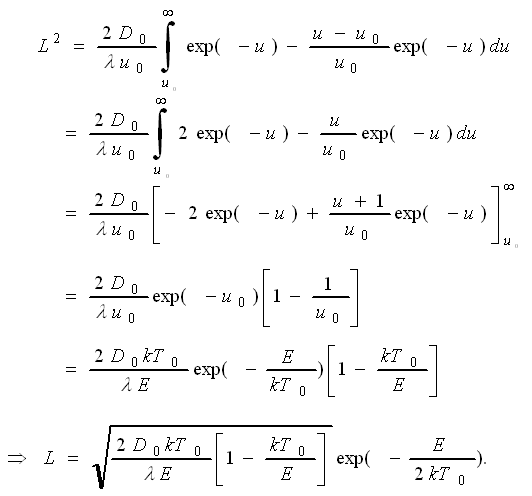
![]() Exercise 4.2-1 Diffusion During Cooling
Exercise 4.2-1 Diffusion During Cooling ![]() Exercise 4.1-1 Lifetime of Positrons
Exercise 4.1-1 Lifetime of Positrons