|
There are several atomic mechanisms
that lead to the movement of atoms. By far the most prominent are the vacancy
mechanism and the direct interstitial mechanism. How they work can be seen in
the animations: |
|
|
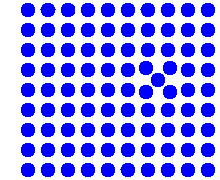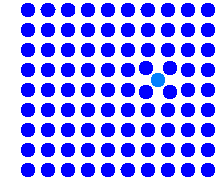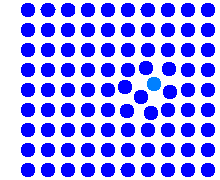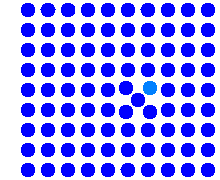
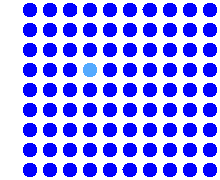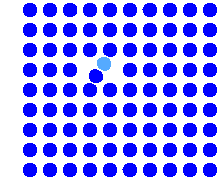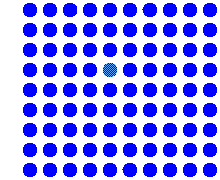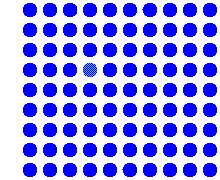
Simulation of the vacancy mechanism |
|
Simulation of the direct interstitial mechanism |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
Note a fundamental difference! If you
consider the diffusion of a particular atom
(any blue one of your choice for the vacancy mechanism, any one of the red ones
for the interstitial mechanism), your selected atom always moved a bit in the second case, but
may not have done anything in the first
case. The diffusion of a particular lattice atom by a vacancy
mechanism, while inextricably linked to the movements of vacancies, is not the
same as vacancy diffusion, but something different! |
|
 |
In other words: if a vacancy has made
N jumps by moving around in the lattice, N atoms
will also have made a jump. However, not necessarily N different atoms, because some individual atoms may have made more than 1
jump. If we look at any particular atom,
there is no way of telling if ti has made a jump or not. At best we can give
some probability. |
|
 |
This leads to a major conclusion:
While the diffusion of a particular lattice
atom by a vacancy mechanism is inextricably linked to the movements of many vacancies, its specific movement is principally
different from the movement of a single vacancy. |
 |
Other mechanisms which are quite rare
but nonetheless potentially important in semiconductors are: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
The simulation shows the elementary step: A self-interstitial
(shown in light blue for easier identification) pushes a lattice atom into the
interstitial lattice. The net effect is the migration of an self-interstitial
from one interstitial site to an different one. |
 |
The mechanism is totally different from the
regular interstitial mechanism. If in a thought experiment you mark a specific self-interstitial atom (paint it red), it
will move a lot with the direct interstitial mechanism, but hardly at all with
the indirect one. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
Interstitial impurity atoms move rather fast by a direct
interstitial mechanism, until they eventually displace a lattice atom. This is
shown in the simulation. We now have a self-interstitial (that may or may not
be very mobile) and a rather immobile substitutional impurity atom, which may
now diffuse with one of the other (slow) mechanisms.
|
 |
The total effect of the diffusion now is caused by the
superpositon of two (usually very
different) mechanisms. Au in Si, and possibly some other
impurities, diffuse in this fashion. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
This is the pendant to the kick-out mechanism. Here the
diffusing impurity atom does not dislodge a lattice atom, but gets trapped in a
vacancy, whereupon it is almost immobile. The total effect may be quite similar
to the kick-out mechanism. |
 |
Which mechanism - Frank-Turnbull or kick-out - is operative,
is difficult to find out. We must expect that in materials containing
predominantly vacancies, the Frank-Turnbull mechanism will occur for some
impurities, while the kick-out mechanism may be operative in materials with
sizeable concentrations of interstitials. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
Shown is one variant, a
direct exchange of places between two atoms. Other variants are exchanges
involving more that 2 atoms (a whole "ring" that
"rotates"). |
 |
Direct mechanisms are every now and then suggested in the
literature to account for some new diffusion phenomena, but so far do not seem
to occur in crystals. |
 |
They may, however, play a role (in analogous form) when
considering diffusion in amorphous materials. |
|
|
|
|
|
 |
Then we could have: |
|
 |
The "Extended
interstitial" mechanism
This is a possibility not yet discussed or observed. It is mentioned just to
show that there might be more atomic mechanisms than have been discovered so
far. Imagine an extended interstitial moving
through a crystal. The 10 or so atoms "inside" the extended
interstitial move around a bit while the interstitial passes through and may
end up on lattice places different from the ones where they were - they have
moved! It is totally unknown if this effect plays a role in Si, but it
well might occur at high temperatures. |
|
 |
And, maybe, there could be more? |
 |
Again, it is important to keep in
mind that you must clearly keep apart the movement of the "vehicle" -
the vacancy, interstitial, etc. - and the movement of the atom(s) whose
diffusion is of interest to you! |
|
|
© H. Föll